- 殷墟發(fā)掘九十周年紀(jì)念大會(huì)
- 安陽(yáng)廣播電視臺(tái)第十七屆豫北春季汽車(chē)文化博覽會(huì)
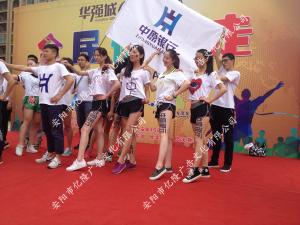
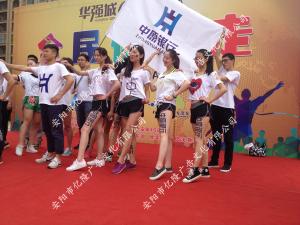
- 華強(qiáng)城房展音樂(lè)節(jié)
- 全國(guó)侵權(quán)盜版及非法出版物集中銷(xiāo)毀活動(dòng)河南分會(huì)場(chǎng)
- 2017年3.15國(guó)際消費(fèi)者權(quán)益保護(hù)紀(jì)念宣傳活動(dòng)
- 安陽(yáng)市春節(jié)電視文藝晚會(huì)現(xiàn)場(chǎng)
- 超級(jí)消防員-全民消防員大型水上競(jìng)技闖關(guān)活動(dòng)
- 運(yùn)動(dòng)會(huì)
- 鶴壁四季青二期開(kāi)業(yè)
- 聚龍國(guó)際酒店開(kāi)業(yè)
- 和美天下城啤酒節(jié)
- 房展華強(qiáng)展區(qū)
- 2013掃黃打非
- 2013年6月房展華強(qiáng)城
- 2012殷商文化節(jié)開(kāi)幕
- 熱烈祝賀億隆廣告文化有限公司網(wǎng)站改版,全新上線(xiàn)!